An Example of a Drug Used to Treat Bph
US Pharm. 2011;36(6):20-24.
Benign prostatic hyperplasia (BPH) is the most common benign tumor in men.1 By age 50 years, up to 50% of men may have histologically distinguished BPH with reported prevalence increasing to 90% by age 90.2 Histology indicating the presence of BPH includes proliferation of prostatic tissue around the urethra. Despite the high prevalence of histologic diagnosis, symptoms of the disorder do not affect all men presenting with BPH. Prostatic tissue overgrowth combined with poor glandular elasticity may lead to urethral opening constriction. This may then become severe enough to negatively affect men's quality of life (QOL) due to increased frequency of BPH-associated urinary symptoms. It has been reported that 50% of men diagnosed with histologic changes indicating BPH demonstrate urinary symptoms at age 80 years.3
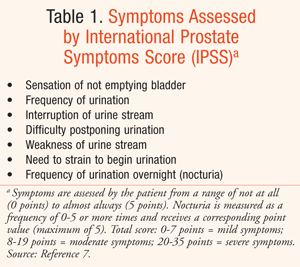
Symptomatic BPH is generally recognized as including the presence of lower urinary tract symptoms (LUTS). These symptoms may include problems with either storage or ability to void urine, or both.3 Symptoms are progressive with age and become significant if prostatic tissue growth results in urethral constriction.3 Symptoms are often measured with a tool developed by the American Urological Association (AUA), known as the International Prostate Symptoms Score (IPSS). TABLE 1 describes symptoms of BPH assessed by this tool, which are related to both storage and voiding dysfunction.
Two main mechanisms can cause bladder obstruction. With prostatic tissue growth, the prostate becomes enlarged. This, in turn, results in a narrowing of the urethral lumen.4 A second mechanism is related to smooth muscle tone mediated by alpha-adrenergic receptors.4 This smooth muscle encapsulates the prostate, urethra, and prostate stoma. Overstimulation of alpha-adrenergic receptors located within the smooth muscle can increase muscle tone, worsening symptoms of obstruction and retention of urine and decreasing the urinary flow rate.4 Commonly used drug classes for BPH (alpha-antagonists, 5-alpha-reductase inhibitors) focus on reversing these mechanisms.
Serious complications can arise from untreated BPH. Acute urinary retention, nephropathy, infections, and bladder stones have all been reported outcomes of uncontrolled disease.2 In addition, much QOL research on BPH management has been done. Severity of symptoms is commonly related to QOL.2 Men with BPH report having concerns over sleep, their condition, mobility, leisure, activities of daily living, and sexual activities.2 Goals of therapy include measureable improvement in QOL through use of the IPSS, amelioration of LUTS, and prevention of serious complications.5
Despite the great prevalence of BPH in today's society, many patients remain untreated. In an analysis of 1,500 men with BPH surveyed in a 1999 study completed in the United Kingdom, only 11% were aware of treatment options that could treat or manage their disease.6 Due to the vital role pharmacists play on the health care team, involvement in the education of patients with BPH is essential in promotion of therapy to manage their disease appropriately. As accessible health care professionals, pharmacists are also well suited to recognize urinary symptoms as cause for alarm and advocate for referral to a health care provider for evaluation to rule out other possible diagnoses (i.e., prostatitis, prostate cancer).
Medication Management of BPH
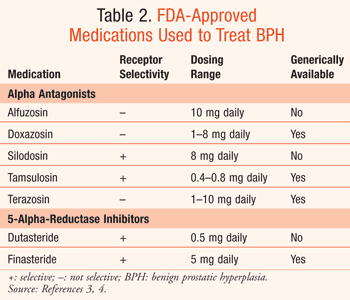
The use of drugs in the treatment of BPH is based upon disease severity as well as the underlying cause. As previously mentioned, the AUA's IPSS can stage a patient's disease. For mild-to-moderate symptoms, a period of watchful waiting is sometimes recommended in which no pharmacotherapy is prescribed. As symptoms progress, alpha-antagonists are typically the first-line agents due to their efficacy and rapid response seen upon initiation.4 If symptoms persist despite optimization of alpha-antagonists, or a patient is unable to tolerate side effects related to their use, 5-alpha-reductase inhibitors may be added to the regimen. Other agents have shown possible benefit as well when used in combination with standard treatment, or as monotherapy if patients are unable to tolerate usual treatment. TABLE 2 reviews the available FDA-approved options for treating BPH, their dosing recommendations, and their generic availability.7
Alpha-Antagonists
The five alpha-antagonists currently available in the United States for the treatment of BPH are alfuzosin, doxazosin, silodosin, tamsulosin, and terazosin. Alpha-antagonists are a useful option in the management of moderate-to-severe LUTS secondary to BPH. Alpha-antagonists are the most effective agents used to treat the moderate-to-severe and bothersome symptoms associated with bladder outlet obstruction (BOO) secondary to BPH.4 Symptoms related to urinary storage including frequency, nocturia, urgency, and incontinence are generally reported as the most bothersome with the largest impact on QOL. These symptoms are lessened by the use of alpha-antagonists. The alpha-antagonists reduce BOO by decreasing the alpha-adrenergic tone of the smooth muscle within the prostate.4
There are three subtypes of alpha-receptors identified within the prostate (a1A, a1B, and a1D), with about 70% of the subtypes being a1A.8,9 The a1B subtype is located primarily in the peripheral vasculature and is vital to blood pressure regulation, with very little effect on BPH. All available alpha-antagonists have activity at all receptors, with only two being semiselective for the a1A subtype, tamsulosin and silodosin. Tamsulosin is more selective for all subtypes, but the ratio of selectivity for the a1A subtype to a1B or a1D is greater for silodosin than tamsulosin. Despite this higher receptor affinity, all alpha-antagonists with the exception of silodosin seem to have equal clinical efficacy, though clinical studies comparing all agents are currently lacking.3
As silodosin was approved in 2008, it has only been compared head to head in a 12-week clinical trial with tamsulosin.10 Silodosin was found to be noninferior to tamsulosin; however, it must be noted that the dose of tamsulosin used in the study (0.2 mg/day) is half the dose approved for clinical use in the U.S. (0.4 mg/day). Most of the alpha-antagonists must be titrated slowly to effective doses with the exception of alfuzosin and silodosin, which can be started at their recommended dose on day 1.3
The main difference between the various alpha-antagonists appears to be their side-effect profiles. The second-generation alpha-antagonists (alfuzosin, doxazosin, terazosin) are all likely to have hypotensive and other syncopal events associated with their use, with alfuzosin causing less hypotension than the other two second-generation medications.11 These adverse effects are dose related, so the lowest effective dose should be used. Alfuzosin may also cause abnormal ejaculations; however, the newer generation agents, tamsulosin and silodosin, are more likely to cause abnormal ejaculations, with silodosin causing more retrograde ejaculation than tamsulosin.12,13 Dizziness has been associated with all agents.
Although rare, another adverse effect of the alpha-antagonists is intraoperative floppy iris syndrome (a complication of cataract surgery). When offered alpha-antagonist treatment, men should be asked if cataract surgery is planned, and therapy should be delayed until after the procedure is completed. Tamsulosin has the most reported cases of floppy iris syndrome.14
All agents should be started at the lowest dose if the patient is concurrently taking a phosphodiesterase type 5 (PDE-5) inhibitor and vice versa because of the possibility of a systemic hypotensive reaction with concurrent use.15-17 Alfuzosin and silodosin are contraindicated in use with concurrent potent CYP3A4 inhibitors, and silodosin is not recommended for use with potent P-glycoprotein inhibitors.18,19
5-Alpha-Reductase Inhibitors
The 5-alpha-reductase inhibitor class has been shown to improve LUTS in the presence of BPH.4 Two drugs currently available within this class are finasteride and dutasteride (TABLE 2). These medications differ in the extent that they are able to block the 5-alpha-reducatase conversion of testosterone to dihydrotestosterone (DHT) within the body. By inhibiting the formation of DHT, 5-alpha-reducatase inhibitors are able to mitigate the effects that androgens have on the prostate. Androgenic effects include proliferation of prostate cells, decrease in prostate cell apoptosis, and elevation of rate of angiogenesis within the prostate.3 Finasteride works solely on the 5-alpha-reductase type II enzyme by inhibiting about 70% of the conversion of testosterone to DHT within the body, while dutasteride has been shown to inhibit both 5-alpha-reductase type I and type II, preventing 95% conversion of testosterone to DHT.4 As a result of the high prevalence of 5-alpha-reductase type II enzymes present within the prostate and genital tissue, any clinical benefits seen from dutasteride's inhibition of both 5-alpha-reductase enzymes have not been evident. However, no comparative trials have been reported.20
Meta-analyses have demonstrated an improved IPSS and peak urinary flow rate in patients who have prostate volumes >30 mL upon initiating therapy.21 Individuals with lower prostate volumes have not shown statistically significant improvement in their symptom scores or peak urinary flow rates and will most likely not benefit from a 5-alpha-reductase inhibitor.21 Additionally, when using an agent in this class, if prostate specific antigen (PSA) levels are monitored for the detection of prostate cancer, one must understand that 5-alpha-reductase inhibitors typically decrease a patient's PSA by about 50%.4 Because of delayed prostatic response to this class of medications, after 3 to 6 months of therapy practitioners should double the PSA level drawn if a patient is on a 5-alpha-reductase inhibitor in order to appropriately assess the patient's true level.2 If PSA levels increase or do not follow this trend, further urologic workup is warranted.4 In addition, dutasteride dosage reductions may be warranted in patients concurrently taking CYP3A4 and CYP3A5 inhibitors.22
It should also be noted that 5-alpha-reductase inhibitors differ in their reported length of activity, with finasteride's half-life estimated to be around 6 to 8 hours while dutasteride's is estimated to be 5 weeks.20 Appropriate treatment duration of at least 6 months should be allowed before a response is evaluated. Clinically important adverse effects in this class are decreased libido, decreased semen quantity during ejaculation, and impotence.21 Furthermore, gynecomastia is reported as a potential, though infrequent, adverse event. Finally, it should be noted that all adverse effects have been shown to diminish after the first year of treatment.20
Combination Therapy
The AUA guidelines for BPH state that with an enlarged prostate, the use of a combination alpha-antagonist and 5-alpha-reductase inhibitor is effective and appropriate.3 In short-term studies, the alpha-antagonists were equally efficacious and safe compared with combination therapy but were better than 5-alpha-reductase inhibitor therapy alone.23,24 However, in long-term studies in men with enlarged prostates, combination therapy with both alpha-antagonist and 5-alpha-reductase inhibitor has demonstrated clear benefit when compared to either agent alone in preventing disease progression and improving symptoms.25 In addition, combination therapy significantly reduces the risk of acute urinary retention as well as the need for invasive procedures to correct the disorder.25
Recently, clinical evidence has emerged for using tolterodine, an anticholinergic, in the treatment of overactive bladder and BPH if symptoms are not controlled with monotherapy with an alpha-antagonist. Current literature evaluates combination therapy only with an alpha-antagonist.26 Tolterodine works by blocking the effects of acetylcholine on the central and peripheral nervous system, thus blunting the bladder contraction effects known to occur when acted upon by the cholinergic nervous system.26 Clinical trials have demonstrated improvements in patients who did not have an overactive bladder but suffered from LUTS that were irritative in nature (i.e., frequency, urgency, and nocturia).3 Tolterodine is the only anticholinergic to date that has been studied in combination treatment of BPH.26 It is important to note that tolterodine should only be used in combination with alpha-antagonists in patients with BPH who have a postvoid residual volume of less than 250 to 300 mL because of the risk of urinary retention with anticholinergic medications.3 The most common adverse effect that has been reported with tolterodine for this indication is dry mouth, with a reported incidence of up to 24%.3 Other adverse events described were similar to placebo.3
Complementary and Alternative Medicine
The AUA does not recommend the use of complementary and alternative medicine (CAM) to treat LUTS caused by BPH.3 This is related to the unavailability of clinical research demonstrating clear benefit with its use as well as the lack of standardization of available products.3
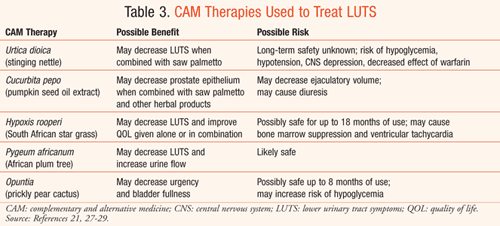
The most well-studied CAM used for treatment of BPH is saw palmetto, which has been described in some clinical trials as "likely safe" and "possibly effective" in treating LUTS associated with BPH.27 In clinical studies, saw palmetto has demonstrated mild improvements in LUTS and urinary flow measures in patients with BPH; however, data from other studies are conflicting, questioning benefit in symptomatic relief with long-term use.4,27,28 Saw palmetto is well tolerated, with minimal adverse effects reported; however, concern over increased risk of bleeding exists with its use, so caution should be used when combining it with other medications that may prolong bleeding time.28 Safety has been established with the use of saw palmetto for up to 1 year.27 Other CAM treatments marketed for LUTS associated with BPH are listed in TABLE 3.21,27-29
Pharmacoeconomic Considerations
Economic considerations include direct medication and resource utilization costs. Other factors considered include severity of symptoms, morbidities resulting from the disease, and patient QOL while on therapy.5 Appropriate patient selection for pharmacotherapy in treating BPH is essential when considering cost of the disease. Watchful waiting is recommended in patients with low symptom severity scores, whereas treatment is indicated in patients who have IPSS scores above 7 and are generally considered to have moderate-to-severe LUTS.5 Symptom severity at this level has demonstrated significantly higher costs, more utilization of health care resources, and decreased QOL of patients, indicating that cost of the disease would outweigh the cost of treatment. Because therapeutic options for treatment of BPH differ in cost, efficacy, and potential for adverse effects, each class must be analyzed separately to determine cost-effectiveness.
Alpha-Antagonists: Though often quite effective in treating LUTS associated with BPH, alpha-antagonists can cause many vasodilatory adverse effects, specifically the second-generation agents.3 Patients taking these types of alpha-antagonists may experience dizziness and hypotension and have increased risk of falling, all of which may negatively impact their QOL in addition to increasing health utilization costs to treat any of these adverse events. However, use of terazosin has demonstrated efficacy in treating LUTS with a significant increase in QOL compared to placebo.30
Alfuzosin and tamsulosin both demonstrate improvement in QOL related to amelioration of LUTS without significant adverse effects. However, compared to earlier-generation alpha-antagonists, the direct cost of these medications is generally higher.5
There are currently few comparisons regarding cost-efficacy analysis within the class; however, a study published in 2004 compared cost-efficacy among tamsulosin, terazosin, and doxazosin use over 3 years.31 Data from the comparison showed that direct medical costs were highest with tamsulosin; however, tamsulosin was also associated with a higher success rate in limiting the need for transurethral resection of the prostate (TURP), demonstrating a large cost savings.31 In addition, at the time of this study, tamsulosin was not generically available.
5-Alpha-Reductase Inhibitors: A number of studies have described the pharmacoeconomic benefit of using finasteride for periods of time less than 3 years.5 When comparing cost efficacy to other therapeutic options, and taking into account the long-term risk reduction provided by 1 year of finasteride therapy, data show finasteride to be less favored than doxazosin, but more cost-efficient than watchful waiting in patients not undergoing surgery.32 Common side effects of sexual dysfunction impair QOL and may impact cost-benefit analysis of these agents, though long-term studies addressing cost efficacy have not been published to date, so it is difficult to make conclusions at this time.
Combination Therapy: Because of its efficacy in halting symptoms and progression of BPH with use of combination therapy, cost-effectiveness has also been demonstrated. Despite the likelihood of sexual side effects with combination therapy and the resultant potential impact on QOL, analytic models demonstrate benefit in men with moderate-to-severe LUTS.32
Conclusion
The medication management of BPH should be focused on improving LUTS associated with the disease while limiting side effects of medications used to treat them. Alpha-antagonists remain the mainstay of therapy, while patients with moderate-to-severe symptoms and enlarged prostates may see additional benefit from 5-alpha-reductase inhibitors. Additional therapies, including herbal remedies and combinations of available pharmacotherapy, may benefit some patients in reducing LUTS.
REFERENCES
1. Scher HI. Chapter 91. Benign and malignant diseases of the prostate. In: Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison's Principles of Internal Medicine. 17th ed. www.accessmedicine.com.cuhsl.
2. McVary KT. BPH: epidemiology and comorbidities. Am J Manag Care. 2006;12(suppl 5):S122-S128.
3. Chapter 1: AUA guideline on the management of benign prostatic hyperplasia: diagnosis and treatment recommendations. American Urological Association.www.auanet.org/
4. Burnett AL, Wein AJ. Benign prostatic hyperplasia in primary care: what you need to know. J Urol. 2006;175:S19-S24.
5. Nickel JC. BPH: costs and treatment outcomes. Am J Manag Care. 2006;12(suppl 5):S141-S148.
6. Trueman P, Hood SC, Nayak US, Mrazek MF. Prevalence of lower urinary tract symptoms and self-reported diagnosed "benign prostatic hyperplasia," and their effect on quality of life in a community-based survey of men in the UK. BJU Int. 1999;83:410-415.
7. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549-1557.
8. Caine M, Raz S, Zeigler M. Adrenergic and cholinergic receptors in the human prostate, prostatic capsule and bladder neck. Br J Urol. 1975;47:193-202.
9. Price DT, Schwinn DA, Lomasney JW, et al. Identification, quantitation, and localization of mRNA for three distinct alpha 1 adrenergic receptor subtypes in human prostate. J Urol. 1993;150:546-551.
10. Kawabe K, Yoshida M, Homma Y, for the Silodosin Clinical Study Group. Silodosin, a new alpha 1A-adrenoreceptor selective antagonist for treating benign prostatic hyperplasia: results of a phase III randomized, placebo-controlled, double-blind study in Japanese men. BJU Int. 2006;98:1019-1024.
11. Lee M. Alfuzosin hydrochloride for the treatment of benign prostatic hyperplasia. Am J Health Syst Pharm. 2003;60:1426-1439.
12. Andersson KE, Gratzke C. Pharmacology of alpha1-adrenoceptor antagonists in the lower urinary tract and central nervous system. Nat Clin Pract Urol. 2007;4:368-378.
13. Van Dijk MM, de la Rosette JJ, Michel MC. Effects of alpha1-adrenoceptor antagonists on male sexual function. Drugs. 2006;66:287-301.
14. Chatziralli IP, Sergentanis TN. Risk factors for intraoperative floppy iris syndrome: a meta-analysis. Ophthalmology. 2011;118:730-735.
15. Viagra (sildenafil) package insert. New York, NY: Pfizer Inc; March 2011.
16. Levitra (vardenafil) package insert. Westhaven, CT: Bayer Healthcare; March 2011.
17. Cialis (tadalafil) package insert. Indianapolis, IN: Eli Lilly; March 2011.
18. Uroxatral (alfuzosin) package insert. New York, NY: Sanofi-aventis Inc; May 2011.
19. Rapaflo (silodosin) package insert. Morristown, NJ: Watson Pharma; May 2011.
20. Kumar VL, Wahane VD. Current status of 5-alpha reductase inhibitors in the treatment of benign hyperplasia of prostate. Indian J Med Sci. 2008;62:167-175.
21. Chapple CR. Pharmacological therapy of benign prostatic hyperplasia/lower urinary tract symptoms: an overview for the practicing clinician. BJU Int. 2004;94:738-744.
22. Dolder CR. Dutasteride: a dual 5-alpha reductase inhibitor for the treatment of symptomatic benign prostatic hyperplasia. Ann Pharmacother. 2006;40:658-665.
23. Lepor H, Williford WO, Barry MJ, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. Veterans Affairs Cooperative Studies Benign Prostatic Hyperplasia Study Group. N Engl J Med. 1996;33:533-539.
24. Kirby RS, Roehrborn C, Boyle P, et al. Efficacy and tolerability of doxazosin and finasteride, alone or in combination, in treatment of symptomatic benign prostatic hyperplasia: the Prospective European Doxazosin and Combination Therapy (PREDICT) trial. Urology. 2003;61:119-126.
25. McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med. 2003;349:2387-2398.
26. Gallegos PJ, Frazee LA. Anticholinergic therapy for lower urinary tract symptoms associated with benign prostatic hyperplasia. Pharmacotherapy. 2008;28:356-365.
27. Natural Medicines Comprehensive Database. http://naturaldatabase.
28. Berardi RR, Ferreri SP, Hume AL, et al. Handbook of Nonprescription Drugs: An Interactive Approach to Self-Care. 16th ed. Washington, DC: American Pharmacists Association; 2009.
29. Levin RM, Das AK. A scientific basis for the therapeutic effects of Pygeum africanum and Serenoa repens. Urol Res. 2000;28:201-209.
30. Hillman AL, Schwartz JS, Willian MK, et al. The cost-effectiveness of terazosin and placebo in the treatment of moderate to severe benign prostatic hyperplasia. Urology. 1996;47:169-178.
31. Ohsfeldt RL, Kreder KJ, Klein RW, Chrischilles EA. Cost-effectiveness of tamsulosin, doxazosin, and terazosin in the treatment of benign prostatic hyperplasia. J Manag Care Pharm. 2004;10:412-422.
32. McDonald H, Hux M, Brisson M, et al. An economic evaluation of doxazosin, finasteride and combination therapy in the treatment of benign prostatic hyperplasia. Can J Urol. 2004;11:2327-2340.
To comment on this article, contact rdavidson@uspharmacist.com.
An Example of a Drug Used to Treat Bph
Source: https://www.uspharmacist.com/article/benign-prostatic-hyperplasia-and-the-medication-management-of-associated-lower-urinary-tract-symptoms
0 Response to "An Example of a Drug Used to Treat Bph"
Post a Comment